 Meet Freya
Meet Freya
If only Regulatory Intelligence was about the proverbial ‘Finding a needle in a haystack.’ A decent metal detector – or specialist database and search engine –and ping, ping, you have your updated guidelines on achieving Market Authorisations for paediatric drugs in Brazil.
The challenge for Regulatory Affairs professionals is not finding needles, it is finding the right needle. Now that almost every article, white paper, guideline, and Royal Decree*, (seriously) – is digitized and online, a combination of the right tools and strategy is needed to navigate through a very complex and always changing regulatory landscape. This article looks at these complexities, shares some of the challenges and questions Freyr consultants get and offers up some approaches you can take.
Try this. Search for ‘Regulations’ on PubMed, FDA.gov and EMA.europa.eu. For the EMA you get 10,077 results relating to ‘humans’ or 20,113 if you include literature sources. The FDA produces 20,098 entries and PubMed is far more complex with 3,201,321 results going back to 1802 and including a vast array of publications, trials, and legislation to name three of the many sub-categories.
All of which leaves us with a central challenge, in Regulatory Intelligence the data is vast, diverse, time-critical, and complex.
At Freyr, Regulatory Intelligence is the backbone of nearly everything we do, from publishing and submissions work through to labelling and PSUR (Periodic Safety Update Report) support services. To achieve 100% compliance and zero recalls, necessitates knowing exactly what local Health Authorities are looking for and what they have updated and released recently. The materials and sources have to be found, read through and considered in detail.
A quarterly review won’t do either. Auditors will not care if your PSUR was missing key data just because your Regulatory Intelligence sources had yet to be updated when you compiled the report. So, one of the first criteria Regulatory professionals need to consider is just how ‘live’ and up to date their data needs to be and how current are their sources?
Freyr is increasingly using AI ‘bots’ and web crawler technology to provide near real-time refreshing of its data sources. The same technologies can also scan, extract and collate all the oncology-related updates in Southeast Asia to compile a report for a particular customers.
What we have learnt over a decade is that the right tools, platforms and sources are only ever half the solution, human expertise and experience – SMEs or Subject Matter Experts – are also vital. To achieve the E, the SMEs ideally need to be local and Freyr works hard to either establish hubs and local representation. Where we cannot have our own people, we set up strategic partnerships with in-country experts. We have branded this affiliate network, calling it FreyrX. Recent projects involving FreyrX include PSUR support, monthly scientific literature searching and MA and MAH (Marketing Authorisation & Marketing Authorisation Holder) support as well as MAH transfers in three countries in the Far East.
So at Freyr, we let the humans and machine do what each does best. Machines are best at repetitive, scalable tasks, where humans can be prone to boredom and making errors. So here we use AI and bots for data collection from websites as well as for data monitoring. Humans – regulatory professionals and SMEs are better at complex tasks and decision making such as data curation, interpretation and deciding what to include and omit from a report.
Different organizations have different needs. So for customers looking for purely Regulatory Intelligence services, we offer three models of delivery. Infrastructure as Service Model – typically best for larger organisations looking to cut back on their digital footprint and too many siloed software tools. Here we deploy Freyr Freya.Intelligence which is tailored to the way the customer works including workflows, data sources and reporting.
Managed Services Model – this is a fully outsourced service where the customer stipulates when kind of report, or insights they are looking for, whether or not it is a one off project or a regular – daily, monthly, quarterly etc. – and Freyr provides the data only.
Custom Solution – typically this will be a combination of the above two. Often Freyr will take on ad-hoc projects with urgent delivery timelines or specialist requirements such as an entirely new market product launch.
Whichever kind of model best suits you, your in-house regulatory team still needs to find the right partner. One that has operational flexibility and can also work the way they work. Many times, we have found that the stakeholders of a new RI platform will either only reluctantly use it, or revert back to Web search and spreadsheets if they feel they have not been listened to and their needs taken into account. This means the partner has to really understand their workflows and processes and be willing and able to configure – at the very least – their platform to accommodate these business needs.
A recent Freya.Intelligence implementation for a global pharma group with multiple stakeholder groups required a partnership development of a Policies module with their own workflows and reporting functions built-in. Benchmarking and highlighting RI findings against their internal Policies and SOPs was vital to company. The good news for other customers is that new and innovative functionality developed for a particular configuration or customisation effort will invariably find its way into a general release update.
Regulatory Intelligence pulls in data and insights from many sources. It is processed, manipulated and pushed out into a multitude of formats for consumption by different stakeholders. This could be senior management looking for new markets to launch an existing drug, or a Safety team needing to include every Rheumatology scientific paper and citation for inclusion in a PSUR report to ensure compliance in an upcoming audit.
Freyr understands that data has multiple dimensions, which we cover, but curate, based on client needs. The data can include Geography, Regulatory Functions, Product Types, diverse HA Sources and then the reporting can vary in terms of level of granularity.
The business requirements are also complex. Effective dashboards are useful for users who need a really clear overview of what is happening in their world. Then the functionality needs to offer the ability to almost endlessly sort, filter and drill-down to the individual paper, HA update or data set they need. They may also want to cross reference results with their internal data.
At Freyr we are wary of single tools claiming to provide a one-stop-shop type solution for Regulatory Intelligence. Think of the sources. As well as the main free industry sites from the FDA, EMA, MHRA and all the individual country HA sites, there are a myriad of subscription sources from providers offering collections of HA publications, white papers, citations, patent data and more. Cortellis, Citeline and Tarius to name a few. Finally, companies have their own internal datasets. Policies, local and regional market intelligence, TA data and competitive commercial insights.
Which means Regulatory teams need to be asking themselves two fundamental data-related questions. First, what sources do they need in terms of geography, therapy area and detail of coverage? Second, how granular and specific do they need the results of their RI search to be?
To meet these needs we developed Freya.Intelligence with an open Cloud based architecture. Very secure access is partnered with easy-to-import capabilities. So searching records from free to source sites such as the EMA is as straightforward as pulling data from subscriptions services and other platforms. Our wide and growing library of industry APIs enables this capability. Freyr believes that this platform approach is the right one, but equally understands we are part of a wider ecosystem that we need to interoperate with.
Whether you are looking for an entirely new platform, or you feel outsourcing and paying for an RI data better suits your needs, or a mix of these two, the overall solution should deliver real, measurable benefits.
Regulatory intelligence, like other domains in Life Sciences, is by its nature made up of disparate silos of data and processes. External databases output CSV files which in turn are filtered and sorted, invariably manually, and then reimported into other systems. And so on. In a recent Freya.Intelligence implementation, we replaced a complex end-of-life RI ecosystem which was made up of SharePoint applications as well as tools including Excel, Word & PowerPoint templates – over 15 separate tools in all. So, our lesson is not to add complexity. Instead, look to simplify, streamline and speed up.
So, your measurement and success criteria could be as simple as ‘we want to replace all our applications and data sources with one platform’. Success criteria and the ones we recommend you consider focus on the following:
I am going to end with a simple graphical showcase, based on a real customer implementation and value or savings that can be made. More importantly, this illustrates how it is possible to achieve near real-time global regulatory compliance.
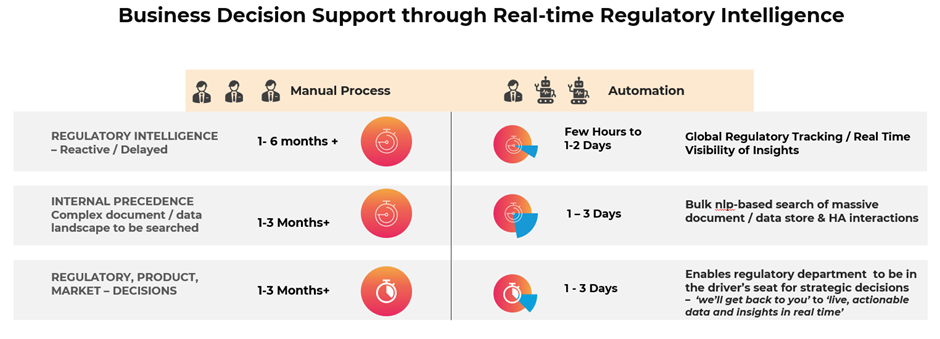
We have similar showcase examples in other service areas including scientific literature review and even labelling with time and cost savings of up to 60%.
Freyr’s platform approach to Regulatory Intelligence – Freya.Intelligence – reimagines and applies a smarter use of visualization, dashboards, analytics and reporting. The most important thing is partnering with our clients to understand their processes, business needs, the data they want to access, inside and outside the business, and finally delivering back a faster, more cost-effective solution.


Reach out to us and let’s unravel your Regulatory puzzle together. Unlock regulatory solutions with our global subject matter experts and tap into a wealth of regulatory intelligence.
Speak to an Expert